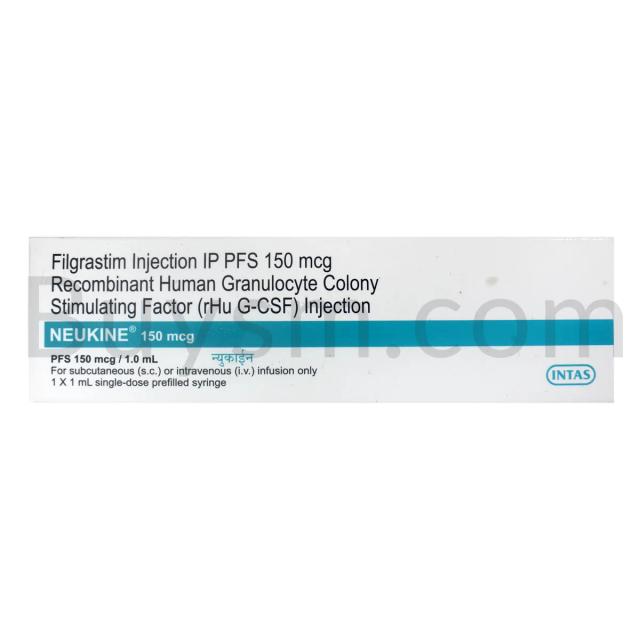
Factocel VIII 250 IU Injection | 250 IU in 1 Vial
- Manufacturer/Marketed By
Intas Pharmaceuticals Ltd
- Active Pharmaceutical Ingredient
Human Anti Haemophilic Factor VIII 250 IU
(Inclusive of all taxes)
Introduction of Factocel VIII 250 IU Injection
Factocel VIII 250 IU Injection is an antihemophilic factor derived from human plasma and is used to control and prevent bleeding episodes or to aid in emergency and elective surgery in adult and pediatric patients with haemophilia A (inherited Factor VIII deficiency). This medicine is not indicated for the treatment of von Willebrand disease (a bleeding disorder caused by low levels of a clotting protein in the blood.
Factocel VIII Injection may cause some common side effects including nervousness, headache, abdominal pain, nausea, numbness and blurred vision, angioedema, burning and stinging at the infusion site, chills, flushing, generalised urticaria, hives, hypotension, lethargy, restlessness, tachycardia, tightness in the chest, tingling, vomiting, wheezing. These side results may be temporary and commonly leave on their own. However, if they persist or get worse, tell your doctor.
Factocel VIII 250 IU is not recommended if you are allergic to any of the ingredients or to mouse or hamster proteins. Tell your doctor about all your medical conditions and if you are pregnant or planning to become pregnant, or are breast-feeding or planning to breast-feed. Also tell your prescriber or health care professional about all other medicines you are taking, including non-prescription medicines, vitamins, or herbal supplements
nti-Hemophilic Drug Factocel VIII is given in a hospital or clinic by a doctor or nurse. Your doctor will decide when to give the injection, so always follow your doctor's advice.The dose and the way regularly you're taking it's going to rely on what you're taking it for. You may also be given other medicines as part of your treatment.You need to take this medicinal drug for so long as it's far prescribed.
Uses of Factocel VIII 250 IU Injection
Prevents or Controls Bleeding In Patients With Hemophilia A: Factocel VIII 250 IU Injection contains human coagulation factor VIII. It is used to treat haemophilia (haemophilia A). Hemophilia A is an inherited bleeding disorder that prevents blood from clotting normally due to a lack of clotting factor, Factor VIII. This Injection is used to control or prevent bleeding episodes in individuals with haemophilia. Factocel VIII 250 IU also helps to perform surgery on hemophilic individuals.
Benefits of Factocel VIII 250 IU Injection
Prevents or Controls Bleeding In Patients With Hemophilia A: The use of Factocel VIII 250 IU Injection has proven to be effective in addressing the deficiency of circulating factor VIII in patients with haemophilia A, a sex-linked hereditary disorder of blood coagulation. This disorder causes a decrease in the level of factor VIII, resulting in excessive bleeding into joints, muscles, or internal organs. Whether it occurs spontaneously or due to accidental or surgical trauma, Factocel VIII 250 IU Injection has demonstrated its ability to prevent or control such bleeding.
Side Effects of Factocel VIII 250 IU Injection
As with all medicines, Factocel VIII 250 IU Vial may have side effects which do not require medical attention and which will disappear as your body adjusts to the medicine. Tell your doctor if they persist or if you're worried about them.
Most Common Side Effects of Factocel VIII 250 IU Injection
- chills
- Flushing
- Generalised urticaria
- Hives
- Hypotension
- Lethargy
- Restlessness
- Tachycardia
- Tightness of the chest
- Tingling
- Vomiting
- Wheezing
Common Side Effects of Factocel VIII 250 IU Injection
- Nervousness
- Headache
- Abdominal pain
- Nausea
- Paresthesia
- Blurred vision
- Angioedema
- Burning and stinging at the infusion site
How to Use Factocel VIII 250 IU Injection?
A doctor or nurse will give you Human Anti-Haemophilic Factor VIII Injection 250 IU in a hospital or clinic. It is important that you always follow your doctor's advice about the timing of your injection. The dose and frequency of administration will depend on the purpose of your treatment. You may also be given other medicines as part of your treatment. It is important that you take all of your medicine for the full prescribed time.
How Factocel VIII 250 IU Injection Works?
Factocel VIII 250 IU Vial contains human coagulation factor VIII. It is essential for blood clotting and haemostasis. Activated Factor VIII (Factor VIIIa) acts as a cofactor for the activation of Factor IX to IXa, which cascades to activate Factor X to Xa. The action of activated Factors Va and Xa converts circulating pro-thrombin to thrombin. Subsequently, thrombin converts fibrinogen to fibrin monomer, cascading to form linear fibrin polymer. Through the action of Factor XIII, the fibrin monomer is cross-linked to form fibrin clots, resulting in the arrest of bleeding episodes.
Safety Advices Factocel VIII 250 IU Injection
Kidney
Not known
No information is not avaliable for this medicine used in kidney disease patients. Please consult your doctor before using this Factocel VIII 250 IU.
Liver
Not known
No information is not avaliable for this medicine used in liver disease patients. Please consult your doctor before using this Factocel VIII Injection.
Driving
Safe
Factocel VIII Injection will not affect your ability to drive or operate machinery. In case you experience any side effects while taking Factocel VIII 250 IU such as dizziness or drowsiness, it is important to avoid driving or operating heavy machinery until the side effects have resolved.
Alcohol
Unsafe
Consumption of alcohol is not recommended while using nti-Hemophilic Drug Factocel VIII. Alcohol may increase or decrease the effectiveness of the medicine and also increase the risk of side effects. It is the best advice for you before consuming alcohol, you can talk with a doctor or pharmacist, and they will tell you consumption of alcohol is safe or not.
Pregnancy
Safe
Factocel VIII 250 IU Injection should be given to a pregnant woman only if clearly needed. Animal reproduction studies have not been conducted with Human Anti-Haemophilic Factor VIII Injection 250 IU. The safety of this medicine use in pregnant women has not been established. It is not known whether Factocel VIII Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity.
Breastfeeding
Safe
Factocel VIII 250 IU Injection should be given to breastfeeding mothers only if clinically indicated. The safety of this medicine used in breastfeeding mothers has not been established. It is not known whether this drug is excreted into human milk. Physicians should carefully consider the potential risks and benefits for each specific patient before prescribing Human Anti-Haemophilic Factor VIII Injection 250 IU.
Similar Medicines
Blood Disorder
Why customers like you choose BuySM.com?

Genuine Medicine
We maintain complete transparency in our sourcing and supply chain processes.

Secure Payments
This ensures that all data exchanged between your browser and our server remains confidential and secure

Fast Delivery
Stay informed about the status of your order with our real-time tracking feature.

Best Price & Offer
Enjoy even greater savings by taking advantage of our bundle offers.

5,000
Total Customers

15,000+
Products Sold Out

10,000+
Orders Delivered

10+
Cities